Gut Feelings: How Microbes and Molecules Shape Digestion, Mood, and Immunity
By Dr Bill Frank, Associate Professor; Executive President – Humanity Gifts Registry (HGR);
Department of Neurobiology and Anatomy; Drexel University College of Medicine, West Reading Campus
Introduction
We often think of the gut as just a place where food is digested. But science is rapidly showing that our digestive tract is a hub of communication, immunity, and even mood regulation. The microbes that live in our intestines don’t just help break down meals – they release powerful chemical messengers that can influence the brain, while immune signals in the gut can fuel or calm inflammation throughout the body. Two areas of cutting-edge research – the new field of neuromicrobiology1 and the role of inflammatory molecules like IL-1β in gut disease2 – are changing how we think about digestion and overall health.
The Gut-Brain Conversation: Neuromicrobiology
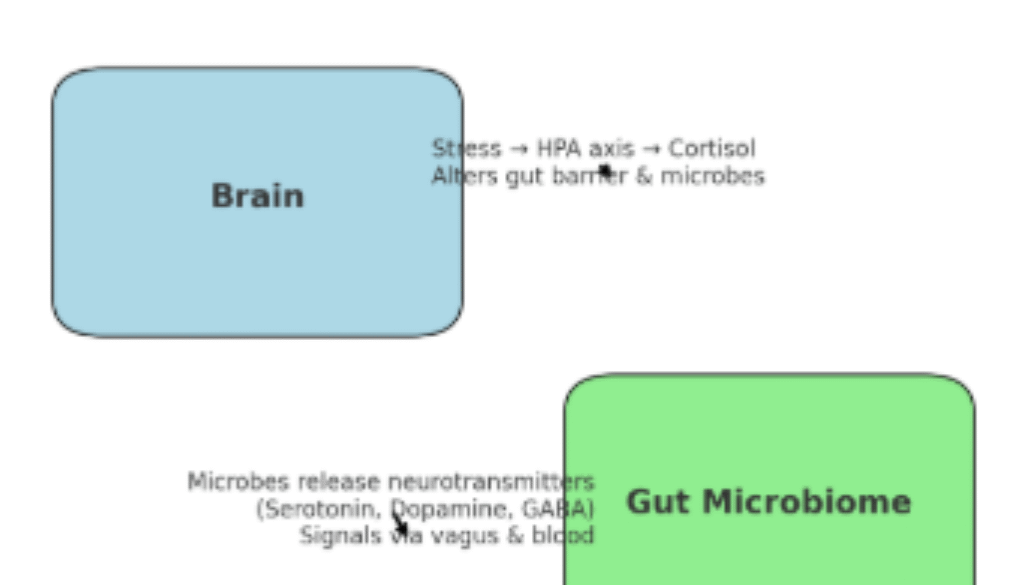
Researchers now know that gut microbes make neuroactive compounds, including serotonin, dopamine, and GABA – chemicals we normally associate with brain function (Figure 1 B.). These molecules can act locally on the intestinal nervous system, travel through the vagus nerve, or even enter circulation to influence mood, memory, and stress.
Communication goes both ways: stress hormones and signals from the brain can also alter gut barrier function and reshape the microbiome (Figure 1 A.). This “two-way street” is at the core of neuromicrobiology, a field exploring how our gut bugs talk to our nervous system and vice versa. Early studies suggest that imbalances in this crosstalk may contribute to conditions ranging from anxiety and depression to irritable bowel syndrome (IBS).
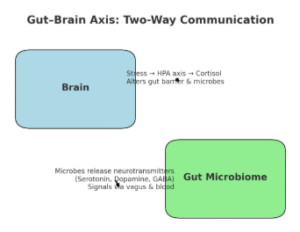
Figure 1. Stress affects your gut. Gut microbes affect your mind. A. Top-Down Pathway (Brain → Gut): Stress → HPA axis → cortisol release → cortisol impacts gut barrier + microbiome balance; B. Bottom-Up Pathway (Gut → Brain): Microbes release serotonin, dopamine, and GABA → signals travel via vagus nerve, bloodstream, and immune messengers
When Gut Balance Breaks: Inflammation and IL-1β
Alongside this chemical chatter, another set of signals controls the immune balance in our intestines. A molecule called IL-1β (interleukin-1 beta) plays a central role in inflammation. In healthy situations, IL-1β helps the body fight infections (Figure 1 A.). But in diseases like Crohn’s disease and ulcerative colitis — the two main types of inflammatory bowel disease (IBD) – IL-1β levels become too high, fueling gut wall damage and chronic inflammation (Figure 1 B.).
Researchers have found that IL-1β is switched on through a protein complex called the NLRP3 inflammasome. This pathway also activates IL-18, another inflammatory signal, and together they drive much of the intestinal damage seen in IBD. Targeting IL-1β, either with natural inhibitors or drugs like anakinra (an IL-1 receptor blocker), may provide new treatment options for people who don’t respond to standard therapies.
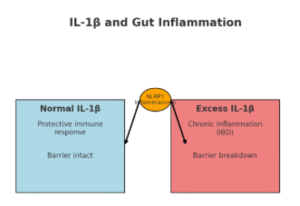
Figure 2. When Gut Immunity Goes Into Overdrive: A. normal IL-1β → controlled, protective response = barrier remains intact; B. Excess IL-1β → chronic inflammation = barrier breakdown. The NLRP3 inflammasome at the center is driving IBD-related inflammation.
Microbes, Mood, and Medicine: The Future of Gut Health
What’s most exciting is the possibility that these two areas of research — microbial metabolites and immune mediators — could one day merge into new therapies. Imagine a probiotic designed not just to aid digestion, but to release neurotransmitters that calm stress or reduce inflammatory cascades in the gut. Or medicines that block IL-1β while preserving the healthy microbial signals that maintain gut balance.
We’re not there yet — but every new discovery shows just how central the gut is to our overall health.
Conclusion
Gut health is about more than digestion. It’s about a finely tuned balance between the trillions of microbes in our intestines, the chemical messages they send, and the immune responses that protect us. As we learn more about neuromicrobiology and inflammatory drivers like IL-1β, we move closer to therapies that treat not just symptoms but the root causes of gut-brain and gut-immune imbalance.
Your gut is talking — and science is finally learning how to listen.
Resources
- Miri S, Yeo J, Abubaker S and Hammami R (2023) Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 14:1098412.doi: 10.3389/fmicb.2023.1098412
- Aggeletopoulou I, Kalafateli M, Tsounis EP and Triantos C (2024) Exploring the role of IL-1β in inflammatory bowel disease pathogenesis. Front. Med. 11:1307394. doi: 10.3389/fmed.2024.1307394
Bio:
Dr. Patrick W. (Bill) Frank is a chiropractic physician and educator with a passion for the gut and microbiome and how they shape overall health and wellness. After more than 20 years of private practice, he now serves as an Associate Professor of Neurobiology & Anatomy at Drexel University College of Medicine. His work bridges clinical experience, teaching, and research, with a special focus on how understanding the gut—and its powerful connection to the brain—can inspire healthier lives.